Monocrystalline silicon, Polycrystalline silicon, and Thin film
PV Cells
Solar PV Cells
A photovoltaic (PV) cell is an energy harvesting technology,
that converts solar energy into useful electricity through a process called the
photovoltaic effect. There are several different types of PV cells which all
use semiconductors to interact with incoming photons from the Sun in order to
generate an electric current.
Layers of a PV Cell
A photovoltaic cell is comprised of many layers of
materials, each with a specific purpose. The most important layer of a
photovoltaic cell is the specially treated semiconductor layer. It is comprised
of two distinct layers (p-type and n-type—see Figure 3), and is what actually
converts the Sun's energy into useful electricity through a process called the
photovoltaic effect (see below). On either side of the semiconductor is a layer
of conducting material which "collects" the electricity produced.
Note that the backside or shaded side of the cell can afford to be completely
covered in the conductor, whereas the front or illuminated side must use the
conductors sparingly to avoid blocking too much of the Sun's radiation from
reaching the semiconductor. The final layer which is applied only to the
illuminated side of the cell is the anti-reflection coating. Since all
semiconductors are naturally reflective, reflection loss can be significant.
The solution is to use one or several layers of an anti-reflection coating
(similar to those used for eyeglasses and cameras) to reduce the amount of
solar radiation that is reflected off the surface of the cell.[2]

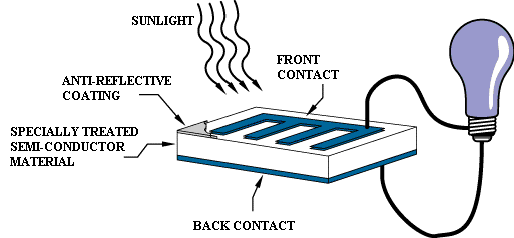
Figure 2. The basic operation of a PV cell.[3]
Photovoltaic Effect
main article
Figure 3. A diagram showing the photovoltaic effect.[4]
The photovoltaic effect is a process that generates voltage
or electric current in a photovoltaic cell when it is exposed to sunlight.
These solar cells are composed of two different types of semiconductors—a
p-type and an n-type—that are joined together to create a p-n junction. By
joining these two types of semiconductors, an electric field is formed in the
region of the junction as electrons move to the positive p-side and holes move
to the negative n-side. This field causes negatively charged particles to move
in one direction and positively charged particles in the other direction.[5]
Light is composed of photons, which are simply small bundles of electromagnetic
radiation or energy. When light of a suitable wavelength is incident on these
cells, energy from the photon is transferred to an electron of the
semiconducting material, causing it to jump to a higher energy state known as
the conduction band. In their excited state in the conduction band, these
electrons are free to move through the material, and it is this motion of the
electron that creates an electric current in the cell.
Solar Cell Efficiency
Efficiency is a design concern for photovoltaic cells, as
there are many factors that limit their efficiency. The main factor is that 1/4
of the solar energy to the Earth cannot be converted into electricity by a
silicon semiconductor. The physics of semiconductors requires a minimum photon
energy to remove an electron from a crystal structure, known as the band-gap
energy. If a photon has less energy than the band-gap, the photon gets absorbed
as thermal energy. For silicon, the band-gap energy is 1.12 electron volts.[6]
Since the energy in the photons from the sun cover a wide range of energies,
some of the incoming energy from the Sun does not have enough energy to knock
off an electron in a silicon PV cell. Even from the light that can be absorbed,
there is still a problem. Any energy above the band-gap energy will be
transformed into heat. This also cuts the efficiency because that heat energy
is not being used for any useful task.[6] Of the electrons that are made
available, not all of them will actually make it to the metal contact and
generate electricity. Some electrons will not be accelerated sufficiently by
the voltage inside the semiconductor to leave the system. These effects combine
to create a theoretical efficiency of silicon PV cells is about 33%.[6]
There are ways to improve the efficiency of PV cells, all of
which come with an increased cost. Some of these methods include increasing the
purity of the semiconductor, using a more efficient semiconducting material
such as Gallium Arsenide, by adding additional layers or p-n junctions to the
cell, or by concentrating the Sun's energy using concentrated photovoltaics. On
the other hand, PV cells will also degrade, outputting less energy over time,
due to a variety of factors including UV exposure and weather cycles. A
comprehensive report from the National Renewable Energy Laboratory (NREL)
states that the median degradation rate is 0.5% per year.[7]
Types of PV Cells
main article
Figure 4. An image comparing a polycrystalline silicon cell
(left) and a monocrystalline silicon cell (right).[8]
Photovoltaic cell can be manufactured in a variety of ways
and from many different materials. The most common material for commercial
solar cell construction is Silicon (Si), but others include Gallium Arsenide
(GaAs), Cadmium Telluride (CdTe) and Copper Indium Gallium Selenide (CIGS).
Solar cells can be constructed from brittle crystalline structures (Si, GaAs)
or as flexible thin-film cells (Si, CdTe, CIGS). Crystalline solar cells can be
further classified into two categories—monocrystalline and polycrystalline, as
shown in figure 4. As the names suggest, monocrystalline PV cells are comprised
of a uniform or single crystal lattice, whereas polycrystalline cells contain
different or varied crystal structures. Solar cells can also be classified by
their number of layers or "p-n junctions". Most commercial PV cells
are only single-junction, but multi-junction PV cells have also been developed
which provide higher efficiencies at a greater cost.
For Further Reading
Photovoltaic effect
Semiconductor
P-n junction
Photon
Types of PV cells
Or explore a random page
References
"20110504-RD-LSC-0621 - Flickr -
USDAgov" by U.S. Department of Agriculture. Licensed under CC BY 2.0 via
Wikimedia Commons -
http://commons.wikimedia.org/wiki/File:20110504-RD-LSC-0621_-_Flickr_-_USDAgov.jpg#/media/File:20110504-RD-LSC-0621_-_Flickr_-_USDAgov.jpg
C. Julian Chen.
Physics of Solar Energy, 1st ed. Hoboken, NJ, USA: John Wiley & Sons Inc.,
2011.
Wikimedia Commons
[Online], Available: http://upload.wikimedia.org/wikipedia/commons/7/7d/Operation_of_a_basic_photovoltaic_cell.gif
Created internally by
a member of the Energy Education team. Adapted from: Ecogreen Electrical.
(August 14, 2015). Solar PV Systems [Online]. Available:
http://www.ecogreenelectrical.com/solar.htm
G. Boyle. Renewable
Energy: Power for a Sustainable Future, 2nd ed. Oxford, UK: Oxford University
Press, 2004.
R. Wolfson,
"Photovoltaic Solar Energy" in Energy, Environment, and Climate, 2nd
ed., New York, NY: W.W. Norton & Company, 2012, ch. 9, sec. 5, pp. 244-252
Dirk C. Jordan and
Sarah R. Kurtz. Photovoltaic Degradation Rates — An Analytical Review, National
Renewable Energy Laboratory, USA, 2012. Accessed April 24, 2018. [Online]
Available at https://www.nrel.gov/docs/fy12osti/51664.pdf
Wikimedia Commons.
(August 18, 2015). Comparison of Solar Cells [Online]. Available:
https://upload.wikimedia.org/wikipedia/commons/7/71/Comparison_solar_cell_poly-Si_vs_mono-Si.png
Authors and Editors
Bethel Afework, Ethan Boechler, Jordan Hanania, Kailyn
Stenhouse, Luisa Vargas Suarez, Brodie Yyelland, Jason Donev
Last updated: December 20, 2021
Get Citation
108 Views | 0 Likes | 0 Comments
© 2025 ENERGPLACE Sdn. bhd. All Rights Reserved.




 Like
Like
 Comment
Comment
 Share
Share